

|
Henry's Law Constantswww.henrys-law.orgRolf Sander |
Atmospheric Chemistry Division Max-Planck Institute for Chemistry |
|---|
HomeHenry's Law ConstantsNotesReferencesDownloadErrataContact, Imprint, AcknowledgementsWhen referring to the compilation of Henry's Law Constants, please cite this publication: R. Sander: Compilation of Henry's law constants (version 5.0.0) for water as solvent, Atmos. Chem. Phys., 23, 10901-12440 (2023), doi:10.5194/acp-23-10901-2023 The publication from 2023 replaces that from 2015, which is now obsolete. Please do not cite the old paper anymore. |
Scientific backgroundHenry's law was formulated by the English chemist William Henry in the early 19th century. It states that the amount of dissolved gas is proportional to its partial pressure in the gas phase. The proportionality factor is called the Henry's law constant.The databaseA compilation of 46434 Henry's law constants for 10173 organic and inorganic species in water was collected from 995 references by Sander (2023). Online access to the searchable database is available here.
Symbols, definitions, and unitsIUPAC recommends eight variants of Henry's law constants (Sander et al., 2022):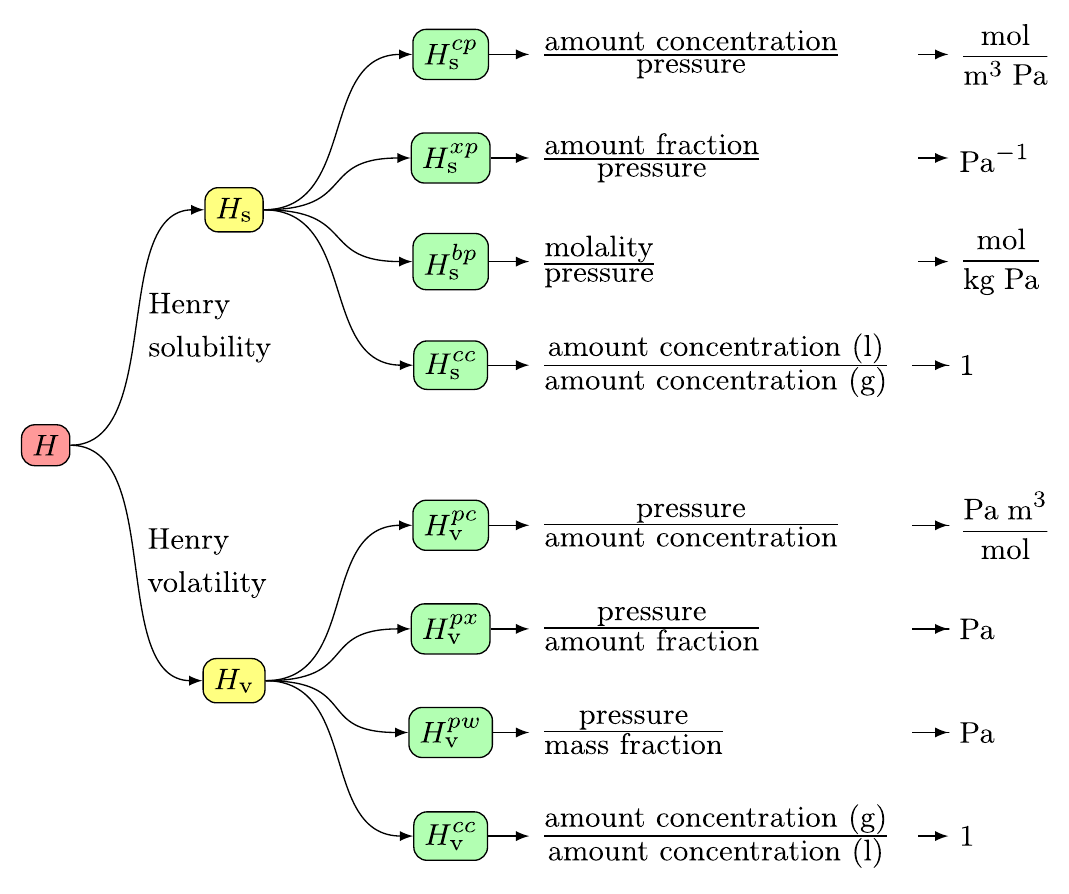 Here, the Henry's law solubility constant is used. It is defined as the ratio of the aqueous-phase concentration of a chemical to its equilibrium partial pressure in the gas phase (at infinite dilution):
To obtain other variants of the Henry's law constant, a conversion tool is available in the right column of this page. What kind of data is not included?
Version HistorySome older (obsolete) versions of the compilation are still available:If you find errors or if you know of additional references that I could include, please send me an email at rolf.sander@mpic.de. If you have published measurements of Henry's law's constants, I would appreciate it very much if you send me a pdf of your paper! |
|